Diethylzinc
Synonym: Zincdiethyl, Zinc diethanide, Zinc ethide, DEZ, DEZn, Et2Zn
CAS Number 557-20-0 | MDL Number MFCD00009021 | EC Number 209-161-3
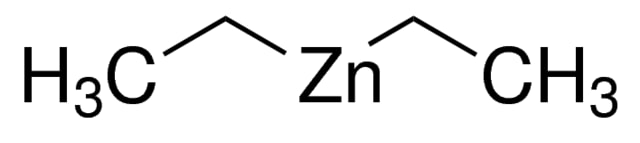
Diethylzinc, also known as zincdiethyl, zinc diethanide, zinc ethide, DEZ, or DEZn, is a highly pure colorless liquid with the molecular formula C₄H₁₀Zn and a molecular weight of 123.5 g/mol. With a purity level of 99%, confirmed by elemental analysis, and a metal purity of 99.9999%-Zn, diethylzinc is a valuable organometallic compound extensively used in various chemical and industrial applications, especially in advanced material production.
Diethylzinc is highly sensitive to moisture and air, requiring careful handling and storage. When exposed to air or water, it reacts vigorously, often leading to the release of flammable gases. As such, diethylzinc must be stored under an inert atmosphere, such as nitrogen or argon, and in tightly sealed containers to prevent contact with moisture and air. Its melting point is -28°C, and it has a boiling point of 124°C, making it a volatile liquid that requires strict temperature control during handling.
This compound is widely used in chemical vapor deposition (CVD) processes, particularly in the production of high-purity zinc-containing films, which are essential in semiconductor manufacturing and optoelectronics. Diethylzinc serves as a precursor for the deposition of zinc oxide (ZnO) thin films, which are critical in producing transparent conductive materials, solar cells, and other advanced technologies.
Diethylzinc is classified as highly flammable and reactive due to its sensitivity to moisture and air. Direct contact can cause severe irritation, burns, and health hazards, and inhalation or ingestion can be dangerous. Proper protective equipment, such as gloves, goggles, and protective clothing, is necessary when handling this compound. It should be used in a well-ventilated area or under fume hoods to prevent exposure to hazardous vapors.
In summary, diethylzinc (DEZ), or zincdiethyl, is a highly reactive, moisture-sensitive liquid used in advanced materials and semiconductor production. Its volatility and hazardous nature demand stringent safety protocols during handling and storage, but its high purity and specific reactivity make it indispensable in the creation of cutting-edge technologies.
Diethylzinc is useful for the deposition of crystalline zinc films by means of plasma-assisted CVD. This product, in combination with molecular oxygen or water, is the most commonly used zinc metal-organic precursor for the growth of ZnO thin films by CVD (including MOCVD, PECVD and ALD. DEZn is also one the most common precursors for p-doping of (Al,Ga)In(As,P) layers grown by MOCVD. Epitaxial layers of Zn-doped InP have been grown by low-pressure MOCVD using this product as the p-dopant source. ZnEt2, in combination with H2S as a sulfur source, has been applied for the ZnS thin films by ALD. See links below.
Diethylzinc is a clear, pyrophoric liquid with a relatively high vapor pressure commonly employed as a volatile source of zinc in a wide range of zinc-containing thin film materials. DEZ is a highly-reactive reagent that is employed broadly in MOCVD, PECVD and ALD processes to prepare numerous compounds, main group, and transition metal elements for simple coatings to electronic and photonic active materials.
Diethylzinc is extremely reactive under some chemical environments, air and water. However, this pyrophoric liquid is routinely safely handled under controlled conditions where these reactants are diligently excluded. A simple distillation under a rigorously-maintained inert nitrogen atmosphere, free of any air or moisture, shows a well-behaved, free-flowing clear liquid, in marked contrast to its behavior in air, as demonstrated in this video.
Diethylzinc is a very useful reagent, widely employed for a number of applications when handled and packaged properly. When working with Diethylzinc, be respectful of its reactivity to prevent fires and possible explosions. The need for safe handling and protection from air, moisture, and other potential reactants is paramount.
Ereztech manufactures and sells this product in small and bulk volumes. Glass ampules, bottles or metal ampules or bubblers are available for packaging. For additional analytical information or details about purchasing ZN7200 contact us at sales@ereztech.com
Safety information
| UN | 3394 |
| Hazardous class | 4.2 (4.3) |
| Packing group | I |
| Pictograms |    |
| Signal word | DANGER |
| Hazard statements | H225-H250-H260-H314-H318-H335-H400-H410 |
| Precautionary statements | P210-P231 + P232-P280-P305 + P351 + P338-P370 + P378-P422 |
| Transport description | ORGANOMETALLIC SUBSTANCE, LIQUID, PYROPHORIC, WATERREACTIVE (Diethylzinc) |
| In TSCA registry | Yes |
Certificates of Analysis (CoA)
If you don’t see the needed lot of Diethylzinc below please contact customer support at sales@ereztech.com
Lot# 4pp Lot# 6pp Lot# 8p Lot# 10p
External identifiers for DEZ
| Pubchem CID | 11185 |
| IUPAC Name | zinc;ethane |
| SMILES | CC[Zn]CC |
| InchI Identifier | InChI=1S/2C2H5.Zn/c2*1-2;/h2*1H2,2H3; |
| InchI Key | HQWPLXHWEZZGKY-UHFFFAOYSA-N |
Known applications and external links
- Jeong Hwan Han, Byoung Kook Lee, Eun Ae Jung, Hyo-Suk Kim, Seong Jun Kim, Chang Gyoun Kim, Taek-Mo Chung, Ki-Seok An. Growth of amorphous zinc tin oxide films using plasma-enhanced atomic layer deposition from bis(1-dimethylamino-2-methyl-2propoxy)tin, diethylzinc, and oxygen plasma. Applied Surface Science Volume 357, Part A, 1 December 2015, Pages 672-677
- Jakob Kuhs, Thomas Dobbelaere. Plasma enhanced atomic layer deposition of zinc sulfide thin films. Journal of Vacuum Science & Technology A 35, 01B111 (2017)
- T.Dobbelaere, M.Minjauw, T.Ahmad, P.M.Vereecken, C.Detavernier. Plasma-enhanced atomic layer deposition of zinc phosphate. Journal of Non-Crystalline Solids Volume 444, 15 July 2016, Pages 43-48
With Diethylzinc other customers often ask:
Ereztech synthesizes and sells additional ZN-compounds.
To purchase ZN7200 contact us at sales@ereztech.com



