Magnesium bromide
Synonym: Magnesium dibromide, Magnesiumbromid
CAS Number 7789-48-2 | MDL Number MFCD00011105 | EC Number 232-170-9
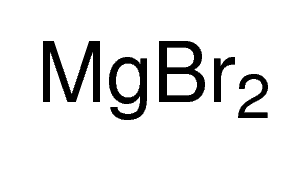
Magnesium bromide, also known as magnesium dibromide, is an inorganic compound with the chemical formula MgBr₂ and a molecular weight of 184.11 g/mol. It appears as an off-white powder and is solid at room temperature. The compound consists of one magnesium atom bonded to two bromine atoms, forming an ionic lattice structure.
Structurally, magnesium bromide is composed of Mg²⁺ cations and Br⁻ anions. The magnesium ion has an oxidation state of +2, and each bromide ion carries a -1 charge, balancing the overall charge of the compound. The ionic bonds result from the significant difference in electronegativity between magnesium and bromine atoms.
It has a high melting point of approximately 711°C, indicative of the strong ionic bonds within its crystal lattice. The compound is highly soluble in water due to its ionic nature, which allows it to dissociate into magnesium and bromide ions readily. It is hygroscopic, meaning it can absorb moisture from the air, so it should be stored in airtight containers to maintain its purity.
Magnesium dibromide is used in organic synthesis as a source of bromide ions. It can serve as a catalyst in various chemical reactions and is instrumental in the preparation of Grignard reagents when reacted with organic halides. These Grignard reagents are essential intermediates in the synthesis of a wide range of organic compounds, including alcohols, acids, and hydrocarbons.
Magnesium bromide should be handled with care. It may cause skin and eye irritation upon contact, as indicated by hazard statements H315 (Causes skin irritation) and H319 (Causes serious eye irritation). Inhalation of dust may lead to respiratory irritation (H335). Appropriate personal protective equipment such as gloves, safety goggles, and dust masks should be used when handling the compound to minimize exposure risks.
The quality and purity of magnesium bromide are critical for its effectiveness in chemical syntheses. The product is typically offered at a purity of 98% or higher, verified by titration methods. Ensuring high purity is essential for laboratory and industrial applications where impurities could affect reaction outcomes.
Magnesium bromide is a valuable inorganic compound in the chemical industry, particularly in organic synthesis. Its ionic properties and reactivity make it a useful reagent for various applications, provided it is stored properly and handled with appropriate safety precautions.
Magnesium bromide is used as a catalyst for many reactions. Such as being a solvent-free one-pot synthesis of dihydropyrimidinones which are used most often in the pharmaceutical industry. MgBr2 in combination with CH2Cl2 catalyzes a reaction that causes specific symmetry and chiral centers through hydrogenation of alkenes. This product, when bonded to other functional groups, has shown more practical uses other than catalyzing reactions. When bonded to an ethyl group it is used for the regiospecific analysis of triglycerols. Magnesium dibromide was also used to synthesize the first stable magnesium silylenoid.
Ereztech manufactures and sells this product in small and bulk volumes. Glass ampules, bottles or metal ampules or bubblers are available for packaging. For additional analytical information or details about purchasing Magnesium bromide contact us at sales@ereztech.com
Safety information
| Signal word | WARNING |
| Pictograms |  |
| Hazard statements | |
| Precautionary statements | P261-P264-P271-P280-P302 + P352-P304 + P340-P305 + P351 + P338-P312-P332 + P313-P337 + P313-P362 |
| In TSCA registry | Yes |
First Aid Measures
| General advice | Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of the dangerous area. |
| Eye contact | Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. |
| Skin contact | Wash off with soap and plenty of water. Consult a physician. |
| Inhalation | If breathed in, move person into the fresh air. If not breathing, give artificial respiration. Consult a physician. |
| If swallowed | Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. |
Certificates of Analysis (CoA)
If you don’t see the needed lot of Magnesium bromide below please contact customer support at sales@ereztech.com
Lot# 031/397 Lot# 023/256 Lot# 023/296 Lot# 007/1278 Lot# 007/ 1278-1 Lot# 007/1338
External identifiers for Magnesium dibromide
| Pubchem CID | 82241 |
| SMILES | [Mg+2].[Br-].[Br-] |
| IUPAC Name | magnesium dibromide |
| InchI Identifier | InChI=1S/2BrH.Mg/h2*1H;/q;;+2/p-2 |
| InchI Key | OTCKOJUMXQWKQG-UHFFFAOYSA-L |
Known applications and external links
With Magnesium bromide other customers often ask:
Ereztech synthesizes and sells additional MG-compounds.
To purchase Magnesium bromide contact us at sales@ereztech.com



