Tin(IV) acetate
Synonym: Stannic Acetate, Tin(4+) Tetraacetate, Tetraacetoxytin, Tin(4+) Diethanoate, Acetic acid tin(IV) salt
CAS Number 2800-96-6 | MDL Number MFCD00014976 | EC Number 628-765-1
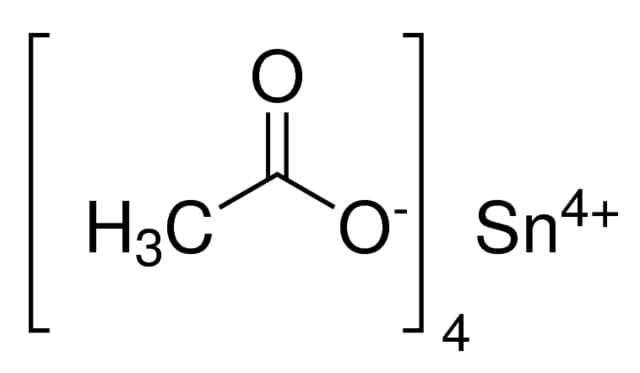
Tin(IV) acetate, also known as stannic acetate or tetraacetoxytin, is a white crystalline powder with the molecular formula C₈H₁₂O₈Sn and a molecular weight of 354.89 g/mol. This compound has a purity of 99%, determined by gravimetric assay, and is widely used in industrial and chemical processes due to its high reactivity and utility in catalysis.
Tin acetate is highly sensitive to both air and moisture, which necessitates careful storage in dry, sealed containers to maintain its stability. Exposure to air or moisture can lead to the decomposition of the compound, affecting its performance in chemical reactions. This moisture sensitivity is particularly important for its use in controlled environments, such as research and development laboratories or industrial applications.
The compound is commonly employed as a catalyst in organic synthesis, particularly in reactions requiring precise control of tin-based intermediates. Its role in catalysis makes it valuable for the production of polymers, coatings, and other advanced materials. Additionally, tin acetate is used as a precursor in the preparation of other tin-containing compounds, broadening its application range.
Tin(IV) acetate is hazardous if inhaled, ingested, or absorbed through the skin, with potential health risks including respiratory and skin irritation (H302, H312, H332). Appropriate safety measures, such as wearing gloves, goggles, and respiratory protection, are recommended when handling the compound. Ensuring proper ventilation is also important to avoid inhaling any dust or fumes.
Tin acetate is a versatile and highly reactive compound used in catalysis and material synthesis. Its sensitivity to air and moisture requires careful handling, but its high purity and specific properties make it an essential material in various industrial and laboratory applications.
Tin(IV) acetate, tin(IV) hydroxide acetate, and tin(IV) chloride pentahydrate have been used as precursors for the precipitation of tin hydroxide and its hydrothermal treatment (HT) to synthesize SnO2. Wurtzite-type copper zinc tin sulfide (Cu2ZnSnS4) nanoparticles were prepared in dodecanethiol (DDT) solutions by reacting corresponding metal acetates with a mixture of sulfur compounds with different reactivities, elemental sulfur (S), and dibutylthiourea (DBTU), via a two-step heat treatment. See links below
Ereztech manufactures and sells this product in small and bulk volumes. Glass ampules, bottles or metal ampules or bubblers are available for packaging. For additional analytical information or details about purchasing SN0966, contact us at sales@ereztech.com
Safety information
| UN | 3146 |
| Hazardous class | 6.1 |
| Packing group | III |
| Pictograms |   |
| Signal word | DANGER |
| Hazard statements | H302-H312-H332 |
| Precautionary statements | P261-P264-P270-P271-P280-P301 + P317-P302 + P352-P304 + P340-P317-P330-P362 + P364-P501 |
| Transport description | ORGANOTIN COMPOUND, SOLID, N.O.S. (Tin(IV) acetate) |
| In TSCA registry | No (research and development usage only) |
First Aid Measures
| General advice | Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of the dangerous area. |
| Eye contact | Rinse thoroughly with plenty of water for at least 15 minutes and get medical help. |
| Skin contact | Wash off with plenty of water. Consult a physician. |
| Inhalation | If breathed in, move person into the fresh air. If not breathing, give artificial respiration. Consult a physician. |
| If swallowed | Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. |
Certificates of Analysis (CoA)
If you don’t see the needed lot of Tin(IV) acetate below please contact customer support at sales@ereztech.com
Lot# 031/324 Lot# 015/748 Lot# 031/311 Lot# 015/836 Lot# 015/872 Lot# 015/904
External identifiers for Tetraacetoxytin
| Pubchem CID | 9863446 |
| IUPAC Name | tin(4+); tetraacetate |
| SMILES | CC(=O)[O-].CC(=O)[O-].CC(=O)[O-].CC(=O)[O-].[Sn+4] |
| InchI Identifier | InChI=1S/4C2H4O2.Sn/c4*1-2(3)4;/h4*1H3,(H,3,4);/q;;;;+4/p-4 |
| InchI Key | YJGJRYWNNHUESM-UHFFFAOYSA-J |
Known applications and external links
- Nishi, H.; Nagano, T.; Kameyama, T.; Kuwabata, S.; Torimoto, T. Well-controlled synthesis of wurtzite-type Cu2ZnSnS4 nanoparticles using multiple sulfur sources via a two-step heating process. CrystEngComm 2015, 17 (1), 174-182.
- Pavelko, R. G.; Yuasa, M.; Kida, T.; Shimanoe, K.; Yamazoe, N. Impurity level in SnO2 materials and its impact on gas sensing properties. Sens. Actuators, B 2015, 210, 719-725.
With Tin(IV) acetate oxide other customers often ask:
Ereztech synthesizes and sells additional SN-compounds.
To purchase SN0966 contact us at sales@ereztech.com



