Trimethyltin chloride
Synonym: Chlorotrimethylstannane, Chlorotrimethyltin, Trimethylchlorotin, Trimethylstannanyl chloride, Trimethyltin monochloride, Me3SnCl
CAS Number 1066-45-1 | MDL Number MFCD00000520 | EC Number 213-917-8
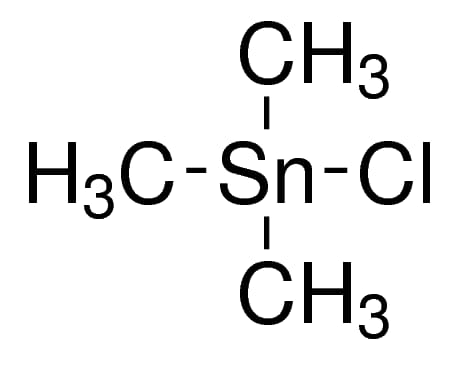
Trimethyltin chloride, also known as Chlorotrimethylstannane or Me₃SnCl, is an organotin compound with the molecular formula C₃H₉ClSn and a molecular weight of 199.27 g/mol. This compound features tin in a +4 oxidation state, coordinated with three methyl groups and a chloride ion, creating a stable, yet reactive, structure. Typically appearing as a colorless liquid or solid, Trimethyltin chloride has a purity of 98%+, verified by gas chromatography. Due to its moisture sensitivity, it should be stored in a dry, sealed container to maintain stability.
Applications in Organic and Inorganic Synthesis
Trimethyltin chloride is commonly used in organotin chemistry and as a precursor for synthesizing various tin-containing compounds. It serves as a reagent in organic synthesis, specifically in processes requiring the transfer of methyl or tin moieties to form complex organometallic structures. Its ability to selectively introduce the trimethyltin group makes it particularly valuable in research, where precise control over synthesis reactions is necessary.
Precautions for Safe Storage and Handling
Classified with hazard codes H300, H310, H315, H319, H330, H335, H336, H373, H400, and H410, Trimethyltin chloride is both highly toxic and potentially hazardous. Protective equipment such as gloves, goggles, and respiratory protection is essential to prevent ingestion, skin contact, or inhalation of fumes. Due to its sensitivity to moisture, the compound should be stored in a dry environment, ideally in tightly sealed containers away from direct sunlight and sources of heat.
Industrial and Laboratory Applications
In industrial and laboratory contexts, Trimethyltin chloride plays a critical role in synthesizing high-purity organotin compounds and is widely applied in material science and chemical research. Its use in producing organotin reagents supports the development of new chemical products and polymers, while its stability under controlled conditions makes it essential in various organometallic transformations. The compound’s unique properties make it highly relevant in the fields of advanced materials, catalyst research, and synthetic chemistry.
Trimethyltin chloride is used as a source of the trimethylstannyl group. Organotin compounds derived from this product are useful in organic synthesis, especially in radical chain reactions. Me3SnCl is also applied as a precursor to compounds used in PVC stabilization. See links below
Ereztech manufactures and sells this product in small and bulk volumes. Glass ampules, bottles or metal ampules or bubblers are available for packaging. For additional analytical information or details about purchasing SN6451 contact us at sales@ereztech.com
Safety information
| UN | 3146 |
| Hazardous class | 6.1 |
| Packing group | I |
| Pictograms |     |
| Signal word | DANGER |
| Hazard statements | H300-H310-H315-H319-H330-H335-H336-H373-H400-H410 |
| Precautionary statements | P260-P262-P264-P270-P271-P273-P280P284-P301 + P310-P302 + P350-P304 + P340-P305 + P351 + P338-P310-P313 + P332-P313 + P337-P330-P361 + P363-P391 |
| Transport description | Organotin compound, solid, n.o.s. (Trimethyltin chloride) |
| In TSCA registry | Yes |
First Aid Measures
| General advice | Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of the dangerous area. |
| Eye contact | Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. |
| Skin contact | Wash off with soap and plenty of water. Consult a physician. |
| Inhalation | If breathed in, move person into the fresh air. If not breathing, give artificial respiration. Consult a physician. |
| If swallowed | Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. |
Certificates of Analysis (CoA)
If you don’t see the needed lot of Trimethyltin chloride below please contact customer support at sales@ereztech.com
Lot# 021/188 Lot# 021/190 Lot# 234R5720 Lot# 34R2421
External identifiers for Chlorotrimethylstannane
| Pubchem CID | 14016 |
| IUPAC Name | chloro(trimethyl)stannane |
| SMILES | C[Sn](C)(C)Cl |
| InchI Identifier | InChI=1S/3CH3.ClH.Sn/h3*1H3;1H;/q;;;;+1/p-1 |
| InchI Key | KWTSZCJMWHGPOS-UHFFFAOYSA-M |
Known applications and external links
- Davies, A. G. (2008). “Tin Organometallics”. Comprehensive Organometallic Chemistry. 3. Elsevier. pp. 809–883.
- Robert J. Morris, Scott L. Shaw, Jesse M. Jefferis, James J. Storhoff, Dean M. Goedde (2007). Monoindenyltrichloride Complexes of Titanium(IV), Zirconium(IV), and Hafnium(IV). Inorg. Synth. Inorganic Syntheses. 32. pp. 215–221.
With Trimethyltin chloride other customers often ask:
Ereztech synthesizes and sells additional SN-compounds.
To purchase SN6451 contact us at sales@ereztech.com



