Vanadium(V) oxytriisopropoxide
Synonym: Triisopropoxyvanadium(V) oxide, VTIP, Vanadium(V) trisisopropoxide oxide, Oxotri(isopropoxo)vanadium, VO(Oi-Pr)3
CAS Number 5588-84-1 | MDL Number MFCD00015017 | EC Number 226-997-4
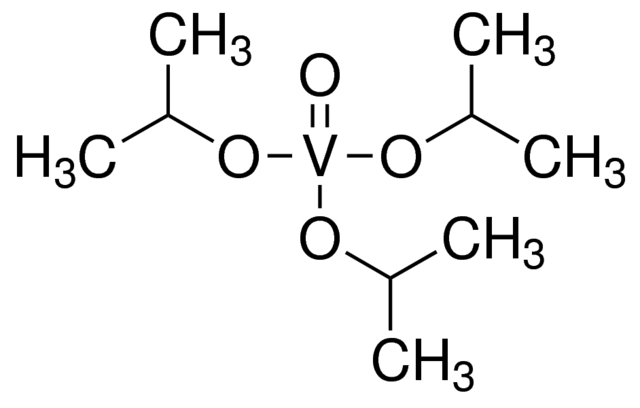
Vanadium(V) oxytriisopropoxide, also known as Oxotris(propan-2-olato)vanadium, is an organovanadium compound with the molecular formula C₉H₂₁O₄V and a molecular weight of 244.2 g/mol. In this structure, vanadium is in the +5 oxidation state, coordinated with one oxo group and three isopropoxide ligands, forming a stable complex. This compound appears as a colorless to yellow-green or gold liquid, with a purity of 98% as verified by titration. Sensitive to air and moisture, it requires careful storage under inert conditions to preserve its reactivity.
Applications in Organic and Inorganic Synthesis
Vanadium(V) oxytriisopropoxide is commonly used as a vanadium source in organic and inorganic synthesis, especially in processes involving oxidation reactions. Its stable coordination makes it highly effective as a precursor in vanadium-catalyzed transformations, including oxidation of alcohols and the synthesis of complex organic molecules. The compound’s reactivity and controlled behavior in catalytic cycles make it suitable for both laboratory research and industrial production of fine chemicals and intermediates.
Precautions for Safe Storage and Handling
This compound is classified with hazard codes H226, H315, H319, and H335, reflecting its flammability and potential for causing skin and eye irritation. Vanadium(V) oxytriisopropoxide is highly sensitive to air and moisture, necessitating storage in sealed containers under an inert gas, such as nitrogen. Handling requires standard safety measures, including gloves, eye protection, and use in well-ventilated areas to avoid inhaling vapors.
Industrial and Laboratory Applications
In industrial and research settings, Vanadium(V) oxytriisopropoxide serves as a reliable vanadium source for applications in catalysis and material synthesis. It plays a significant role in the preparation of vanadium oxides and other high-performance vanadium-based materials. Additionally, it is utilized in semiconductor processes, where controlled vanadium deposition is required. Its versatility and stability make it an essential compound in both the chemical and material sciences industries.
Vanadium(V) oxytriisopropoxide has been applied as a V precursor for the MOCVD growth of V-doped TiO2 thin films to form a high-performance anode for lithium-ion batteries. This product is a building block for a series of neutral oxovanadium complexes containing both ethoxide and acetylacetonate ligands the complexes have potential biological relevance as well as uses in catalysis. See links below
Ereztech manufactures and sells this product in small and bulk volumes. Glass ampules, bottles or metal ampules or bubblers are available for packaging. For additional analytical information or details about purchasing Vanadium(V) oxytriisopropoxide contact us at sales@ereztech.com
Safety information
| UN | 1993 |
| Hazardous class | 3 |
| Packing group | III |
| Pictograms |   |
| Signal word | WARNING |
| Hazard statements | H226-H315-H319-H335 |
| Precautionary statements | P210-P233-P240-P241-P242-P243-P261-P264-P271-P280-P303 + P361 + P353 |
| Transport description | FLAMMABLE LIQUID, N.O.S. (Vanadium(V) triisopropoxide oxide) |
| In TSCA registry | Yes |
First Aid Measures
| General advice | Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of the dangerous area. |
| Eye contact | Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. |
| Skin contact | Wash off with soap and plenty of water. Consult a physician. |
| Inhalation | If breathed in, move person into the fresh air. If not breathing, give artificial respiration. Consult a physician. |
| If swallowed | Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. |
Certificates of Analysis (CoA)
If you don’t see the needed lot of Vanadium(V) oxytriisopropoxide below please contact customer support at sales@ereztech.com
Lot# 018/167 Lot# 032/110 Lot# 032/106
External identifiers for VTIP
| Pubchem CID | 79702 |
| SMILES | CC(C)O.CC(C)O.CC(C)O.O=[V] |
| IUPAC Name | oxovanadium; propan-2-ol |
| InchI Identifier | InChI=1S/3C3H8O.O.V/c3*1-3(2)4;;/h3*3-4H,1-2H3;; |
| InchI Key | JOUSPCDMLWUHSO-UHFFFAOYSA-N |
Known applications and external links
- J. Musschoota,z, D. Deduytschea, H. Poelmana, J. Haemersa, R. L. Van Meirhaeghea, S. Van den Bergheb and C. Detavernie Comparison of Thermal and Plasma-Enhanced ALD/CVD of Vanadium Pentoxide. Journal of The Electrochemical Society, 156 (7) P122-P126 (2009)
- Y. Gao, S. Thevuthasan, D.E. McCready, M. Engelhard, J. Cryst. Growth, 2000, Vol. 212, Iss. 1–2, p.178-190 MOCVD growth and structure of Nb- and V-doped TiO2 on sapphire
With Vanadium(V) oxytriisopropoxide other customers often ask:
Ereztech synthesizes and sells additional V-compounds.
To purchase Vanadium(V) oxytriisopropoxide contact us at sales@ereztech.com



