Dicobalt Octacarbonyl
Synonym: Cobalt carbonyl, Cobalt tetracarbonyl dimer, Octacarbonyldicobalt, Cobalt Octacarbonyl
CAS Number 10210-68-1 | MDL Number MFCD00016024
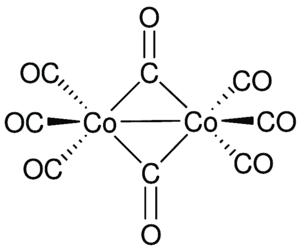
Dicobalt octacarbonyl, also known as cobalt carbonyl, cobalt tetracarbonyl dimer, or octacarbonyldicobalt, is a solid organometallic compound with the molecular formula Co₂(CO)₈ and a molecular weight of 341.95 g/mol. This substance is typically available as orange or dark red crystals and has a purity level of 95%, verified by titration. It is a widely used cobalt-based compound, particularly in catalysis and organic synthesis.
The compound is highly sensitive and requires careful handling due to its reactive nature. Dicobalt octacarbonyl has a melting point of 51-52°C, and it is often stabilized with 1-5% hexane to prevent decomposition. It should be stored under controlled conditions to preserve its stability, typically in a cool, dry place away from sources of moisture or heat. The compound is known for its volatility and toxicity, so storage in sealed containers is necessary to prevent unwanted exposure.
Dicobalt octacarbonyl is a key precursor in the production of cobalt catalysts and is extensively used in hydroformylation reactions. It is valuable in the field of organometallic chemistry, where it facilitates the synthesis of cobalt-containing compounds. Additionally, this compound plays a role in industrial processes, such as the production of fine chemicals and pharmaceuticals, due to its reactivity in forming carbonyl complexes.
Exposure to dicobalt octacarbonyl presents significant health risks. It is classified as highly flammable (H251) and poses severe health hazards if inhaled (H330), ingested (H302), or in contact with skin (H315). It can also cause allergic skin reactions (H317) and is suspected of causing cancer (H351) and reproductive harm (H361). Due to these risks, personal protective equipment, including gloves, goggles, and respirators, is mandatory when handling this compound. Proper ventilation and avoidance of direct contact with the compound are critical to minimizing health hazards.
Dicobalt octacarbonyl is a highly reactive and valuable compound in industrial and chemical synthesis. Its catalytic properties make it indispensable in various high-tech processes, but due to its toxicity and sensitivity, strict safety protocols must be observed during handling and storage.
Dicobalt octacarbonyl is used for atomic layer deposition by semiconductors and related industries. Surfaces for such deposition can include metals, metal nitrides, metal silicides, and metal oxides. This product is valued for its ability to be a precursor for the selective deposition of cobalt-containing films onto specific surfaces, enabling superior film performance regarding continuity, uniformity, and low resistance. In organometallic chemistry and organic synthesis Co2(CO)8 is a reagent and catalyst used for hydroformylation: the conversion of alkenes into aldehydes. This product is highly reactive towards alkynes, so it is sometimes used as an alkyne-protecting compound. Synthesis of this product has been reported (see the links below). Ereztech manufactures and sells Dicobalt Octacarbonyl in small and bulk volumes. Glass ampules and bottles or metal ampules and bubblers are available for packaging. For additional information or details about purchasing Dicobalt Octacarbonyl contact us at sales@ereztech.com
Safety information
| UN | 3190 |
| Hazardous class | 4.2 |
| Packing group | II |
| Pictograms |    |
| Signal word | DANGER |
| Hazard statements | H251-H302-H-304-H315-H317-H330-H351-H361-H412 |
| Precautionary statements | P201-P260-P273-P280-P304 + P340 + P310-P403 + P233 |
| Transport description | SELF-HEATING SOLID, INORGANIC, N.O.S. (Octacarbonyldicobalt) |
| In TSCA registry | Yes |
First Aid Measures
| General advice | Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of the dangerous area. |
| Eye contact | Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. |
| Skin contact | Wash off with soap and plenty of water. Consult a physician. |
| Inhalation | If breathed in, move person into the fresh air. If not breathing, give artificial respiration. Consult a physician. |
| If swallowed | Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. |
Certificates of Analysis (CoA)
If you don’t see the needed lot of Dicobalt octacarbonyl below please contact customer support at sales@ereztech.com
Lot# 005/613Lot# 005/603 Lot# 005/623 Lot# 005/668 Lot# 005/669 Lot# 005/670 Lot# 005/694 Lot# 005/702
External identifiers for Cobalt tetracarbonyl dimer
| Pubchem CID | 25049 |
| SMILES | O=C=[Co]1(=C=O)(=C=O)C(=O)[Co](=C=O)(=C=O)(=C=O)C1=O |
| IUPAC Name | Octacarbonyldicobalt(Co—Co) |
| InchI Identifier | InChI=1S/8CO.2Co/c8*1-2;;/q;;;;;;;;2*+2 |
| InchI Key | MQIKJSYMMJWAMP-UHFFFAOYSA-N |
Known applications and external links
- Pauson, P. L.; Khand, I. U. (1977). “Uses of Cobalt-Carbonyl Acetylene Complexes in Organic Synthesis”. Ann. N. Y. Acad. Sci. 295 (1): 2–14.
- Teobald, Barry J. (2002). “The Nicholas reaction: The use of dicobalt hexacarbonyl-stabilised propargylic cations in synthesis”. Tetrahedron (Review). 58 (21): 4133–4170.
With Dicobalt Octacarbonyl other customers often ask:
- (3,3-Dimethyl-1-butyne)dicobalt hexacarbonyl
- Cobalt tricarbonyl nitrosyl
- Dicarbonylcyclopentadienylcobalt
- Bis(N,N-di-tert-butyl-1,4-diaza-1,3-butadienyl)cobalt
Ereztech synthesizes and sells additional Co-compounds.
To purchase Dicobalt Octacarbonyl contact us at sales@ereztech.com



