Iron(II) acetate
Synonym: Ferrous acetate, Iron diacetate, Fe(OAc)2
CAS Number 3094-87-9 | MDL Number MFCD00058909 | EC Number 221-441-7
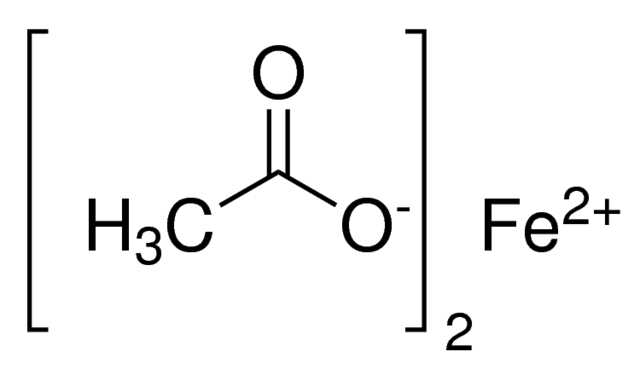
Iron(II) acetate, also known as ferrous acetate or iron diacetate, is an anhydrous iron salt with the molecular formula C₄H₆O₄Fe and a molecular weight of 173.93 g/mol. It appears as a tan to light grey powder and is sensitive to air and moisture.
The compound consists of iron in the +2 oxidation state coordinated to two acetate ions. Its melting point ranges from 190°C to 200°C. Due to its sensitivity to air and moisture, iron(II) acetate should be handled under inert atmospheric conditions and stored in airtight containers to maintain its purity.
Iron(II) acetate is utilized as a precursor in the synthesis of iron-based materials and catalysts. It plays a significant role in the preparation of iron nanoparticles and various coordination compounds. Its applications extend to fields such as material science, catalysis, and nanotechnology.
Handling iron(II) acetate requires caution. It may cause skin irritation (H315), eye irritation (H319), and respiratory irritation (H335). Appropriate personal protective equipment, such as gloves and eye protection, should be used when working with this compound.
The quality and purity of iron(II) acetate are critical for its effectiveness in specialized applications. It is typically offered at a purity of 98% or higher, verified by titration methods, with a metal purity of 99.99% iron content.
Iron(II) acetate serves as an important iron salt in various chemical syntheses and industrial processes. Its properties and high purity make it suitable for advanced research and development in multiple scientific fields.
Iron(II) acetate has been used to synthesize iron oxide nanoparticles which were further used to form iron oxide-poly(ethylene glycol) core-shell nanoparticles (NPs). The core-shell NPs were studied for self-assembly at liquid-liquid interfaces (SALI) forming monolayers. This product is also utilized as a mordant by the dye industry, as a catalyst in organic oxidation reactions and in carbon nanotube synthesis. See links below
Ereztech manufactures and sells this product in small and bulk volumes. Glass ampules, bottles or metal ampules or bubblers are available for packaging. For additional analytical information or details about purchasing Iron(II) acetate contact us at sales@ereztech.com
Safety information
| UN | Not regulated |
| Hazardous class | Not regulated |
| Packing group | Not regulated |
| Pictograms |  |
| Signal word | WARNING |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P264-P271-P280-P302 + P352-P304 + P340-P305 + P351 + P338-P312-P332 + P313-P337 + P313-P362 |
| Transport description | NONH for all modes of transport |
| In TSCA registry | Yes |
First Aid Measures
| General advice | Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of the dangerous area. |
| Eye contact | Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. |
| Skin contact | Wash off with soap and plenty of water. Consult a physician. |
| Inhalation | If breathed in, move person into the fresh air. If not breathing, give artificial respiration. Consult a physician. |
| If swallowed | Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. |
Certificates of Analysis (CoA)
If you don’t see the needed lot of Iron(II) acetate below please contact customer support at sales@ereztech.com
Lot# 029/176 Lot# 032/087 Lot# 007/1234 Lot# 007/1266 Lot# 023/299 Lot# 029/231 Lot# 007/1324 Lot# 007/1335 Lot# 007/1342
External identifiers for Ferrous acetate
| Pubchem CID | 517325 |
| SMILES | [Fe+2].[O-]C(=O)C.[O-]C(=O)C |
| IUPAC Name | acetic acid; iron |
| InchI Identifier | InChI=1S/2C2H4O2.Fe/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2 |
| InchI Key | LNOZJRCUHSPCDZ-UHFFFAOYSA-L |
Known applications and external links
- Katerina Polakova, Jiri Pechousek, Jiri Tucek, Jan Filip, Roman Kubinek, Radek Zboril, Petr Paucek Controlled Solid-State Synthesis of Mri Effective Superparamagnetic Maghemite Nanoparticles from Iron(II) Acetate
- Faure, A. M.; Koppenol, W. H.; Nyström, L. Iron(II) binding by cereal beta-glucan. Carbohydr. Polym. 2015, 115, 739-743.
With Iron(II) acetate other customers often ask:
Ereztech synthesizes and sells additional FE-compounds.
To purchase Iron(II) acetate contact us at sales@ereztech.com



