(Triphenylphosphine)copper hydride hexamer
Synonym: Cuprous hydride triphenylphosphine hexamer, Hydrido(triphenylphosphine)copper(I) hexamer, Triphenylphosphine–Copper(I) hydride Hexamer, Stryker’s reagent
CAS Number 33636-93-0 | MDL Number MFCD00221518
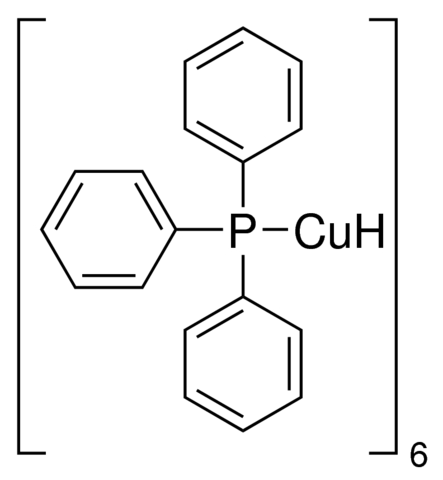
Hydrido(triphenylphosphino)copper(I) hexamer, also known as (Triphenylphosphine)copper hydride hexamer or Stryker’s reagent, is an organocopper compound with the molecular formula C₁₀₈H₉₆Cu₆P₆ and a molecular weight of 1961.04 g/mol. This compound features copper in the +1 oxidation state coordinated with hydride ions and triphenylphosphine ligands in a hexameric arrangement, providing a stable framework for its reducing properties. Appearing as an orange to dark red powder with a purity of 95% (determined by elemental analysis), it is moisture-sensitive and requires storage in a dry environment to maintain stability.
Applications in Organic and Inorganic Synthesis
This compound is a widely-used reducing agent in organic synthesis, particularly for selective reductions of unsaturated carbonyl compounds. Its structure, featuring coordinated copper and triphenylphosphine ligands, provides stability and selectivity, making it ideal for complex synthetic applications.
Precautions for Safe Storage and Handling
Classified with hazard codes H302, H315, H319, and H335, Hydrido(triphenylphosphino)copper(I) hexamer should be handled with care. Standard protective equipment, such as gloves and eye protection, is recommended. Due to its sensitivity, it should be stored in a cool, dry location.
Industrial and Laboratory Applications
Stryker’s reagent is essential in synthetic and pharmaceutical research, where it enables selective reductions in complex organic molecules. It is commonly used for transformations requiring copper-centered catalysis, advancing the development of fine chemicals and specialized materials.
(Triphenylphosphine)copper hydride hexamer is used as a catalyst for conjugate reduction of ortho-substituted cinnamic esters to form copper enolates, chemoselective preparation of alcohols via hydrogenation of aldehydes, 1,2-addition/transmetalation reactions, synthesis of the monomer for preparation of phosphorus- and silicon-containing epoxy resins, hydrosilylation reactions, chiral hydrogenation reactions, hydrostannation of activated alkynes. Some useful synthetic applications of this product include stoichiometric and catalytic hydrogenations, chemoselective conjugate reductions, as well as regiospecific and stereoselectiveconjugate hydride reductions. See link below
Ereztech manufactures and sells this product in small and bulk volumes. Glass ampules, bottles or metal ampules or bubblers are available for packaging. For additional analytical information or details about purchasing (Triphenylphosphine)copper hydride hexamer contact us at sales@ereztech.com
Safety information
| UN | Not regulated |
| Hazardous class | Not applicable |
| Packing group | Not applicable |
| Pictograms |  |
| Signal word | WARNING |
| Hazard statements | H228-H302-H315-H319-H335 |
| Precautionary statements | P261-P264-P270-P271-P280-P301 + P312-P302 + P352-P304 + P340-P305 + P351 + P338-P312-P330-P332 + P313 |
| Transport description | NONH for all modes of transport |
| In TSCA registry | Yes |
First Aid Measures
| General advice | Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of the dangerous area. |
| Eye contact | Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. |
| Skin contact | Wash off with soap and plenty of water. Consult a physician. |
| Inhalation | If breathed in, move person into the fresh air. If not breathing, give artificial respiration. Consult a physician. |
| If swallowed | Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. |
Certificates of Analysis (CoA)
If you don’t see the needed lot of (Triphenylphosphine)copper hydride hexamer below please contact customer support at sales@ereztech.com
Lot# 003/522 Lot# 003/584 Lot# 003/584-1
External identifiers for Stryker’s reagent
| Pubchem CID | 12181933 |
| SMILES | C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.[Cu].[Cu].[Cu].[Cu].[Cu].[Cu] |
| IUPAC Name | copper monohydride; triphenylphosphane |
| InchI Identifier | InChI=1S/6C18H15P.6Cu.6H/c6*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;;;;;;;;;;/h6*1-15H;;;;;;;;;;;; |
| InchI Key | PYHLOCIDGQNZFY-UHFFFAOYSA-N |
Known applications and external links
With (Triphenylphosphine)copper hydride hexamer other customers often ask:
- Bis(dimethylamino-2-propoxy)copper(II)
- Bis(N,N’-di-sec-butylacetamidinato)dicopper(I)
- 2,2,6,6-Tetramethyl-3,5-heptanedionate(II)copper
Ereztech synthesizes and sells additional CU-compounds.
To purchase (Triphenylphosphine)copper hydride hexamer contact us at sales@ereztech.com



