Aluminum chloride
Synonym: Aluminum chloride anhydrous, Aluminum trichloride, AlCl3
CAS Number 7446-70-0 | MDL Number MFCD00003422 | EC Number 231-208-1
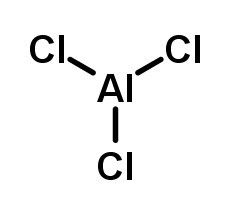
Aluminum chloride, also known as aluminum trichloride or AlCl₃, is a light yellow inorganic compound widely used in the chemical industry. Its molecular formula is AlCl₃, and it has a molecular weight of 133.34 g/mol. The compound consists of one aluminum atom covalently bonded to three chlorine atoms and is highly hygroscopic, reacting readily with moisture.
In solid form, aluminum chloride has a layered crystal structure and exists as dimers (Al₂Cl₆) in the vapor phase. This electron-deficient aluminum center makes it a strong Lewis acid, which is why it is an effective catalyst in numerous chemical reactions. It readily undergoes hydrolysis when exposed to water, producing hydrogen chloride gas and aluminum hydroxide.
Aluminum chloride appears as a light yellow powder and is a solid at room temperature. It has a melting point of approximately 192.4°C and sublimes around 178°C under atmospheric pressure. Due to its hygroscopic nature, it fumes in moist air and must be handled carefully to prevent reaction with atmospheric moisture.
For storage and packaging, aluminum chloride should be kept in airtight, moisture-proof containers to prevent degradation. It is sensitive to moisture and should be stored in a cool, dry place away from sources of water.
The primary application of aluminum chloride is as a catalyst in organic synthesis, particularly in Friedel-Crafts alkylation and acylation reactions. These reactions are essential for forming carbon-carbon bonds in the production of various petrochemicals, pharmaceuticals, and dyes. Additionally, aluminum chloride is used in the manufacturing of lubricants, rubber, wood preservatives, and as a component in some antiperspirants.
Handling aluminum chloride requires caution due to its corrosive properties and reactivity with water. Exposure can cause severe irritation or burns to the skin, eyes, and respiratory tract. In the event of contact, affected areas should be thoroughly rinsed with water. It is important to use appropriate personal protective equipment such as gloves, safety goggles, and protective clothing when working with this compound.
The quality and purity of aluminum chloride are critical for its effectiveness in industrial applications. High-purity grades with 99.999% aluminum content are available, ensuring suitability for sensitive chemical processes where impurities could affect outcomes.
In summary, aluminum chloride is a crucial inorganic compound with significant roles in the chemical industry, especially as a catalyst in organic synthesis. Its strong Lewis acid characteristics make it indispensable in various industrial processes. However, due to its reactivity and corrosiveness, careful handling and storage are imperative to ensure safety and maintain its efficacy.
Aluminium chloride, as one of the most commonly used and also one of the most powerful acid , finds a wide variety of applications in organic chemistry. It can be used to introduce aldehyde groups onto aromatic rings. This product is also finds application in the chemical industry as a catalyst for Friedel–Crafts reactions, both acylations and alkylations. For example in the preparation of anthraquinone (for the dyestuffs industry) from benzene and phosgene. It is also widely used for polymerization and isomerization reactions of hydrocarbons. Important examples include the manufacture of ethylbenzene, which used to make styrene and polystyrene, and also production of dodecylbenzene, which is used for making detergents.
Ereztech manufactures and sells this product in small and bulk volumes. Glass ampules, bottles or metal ampules or bubblers are available for packaging. For additional analytical information or details about purchasing Aluminium chloride contact us at sales@ereztech.com
Safety information
| UN | 1726 |
| Hazardous class | 8 |
| Packing group | II |
| Pictograms |  |
| Signal word | DANGER |
| Hazard statements | H290-H314-H318-H402 |
| Precautionary statements | P234-P260- P264-P280-P301 + P330 + P331-P303 + P361 + P353-P304 + P340-P305 + P351 + P338-P310-P363-P390 |
| Transport description | Aluminum chloride, anhydrous |
| In TSCA registry | Yes |
First Aid Measures
| General advice | Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of dangerous area. |
| Eye contact | Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. |
| Skin contact | Wash off with soap and plenty of water. Consult a physician. |
| Inhalation | If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. |
| If swallowed | Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. |
Certificates of analysis (CoA)
If you don’t see the needed lot of Aluminium chloride below please contact customer support at sales@ereztech.com
External identifiers for Aluminum trichloride
| Pubchem CID | 24012 |
| SMILES | [Al](Cl)(Cl)Cl |
| IUPAC Name | trichloroalumane |
| InchI Identifier | InChI=1S/Al.3ClH/h;3*1H/q+3;;;/p-3 |
| InchI Key | VSCWAEJMTAWNJL-UHFFFAOYSA-K |
Known applications and external links
With Aluminium chloride other customers often ask:
Ereztech synthesizes and sells additional Al-compounds.
To purchase Aluminium chloride CAS 7446-70-0 contact us at sales@ereztech.com



