Hexachlorodisiloxane
Synonym: Hexachloropropanedisiloxane, Perchlorodisiloxane, HCDSO
CAS Number 14986-21-1 | MDL Number MFCD00031530 | EC Number 239-070-4
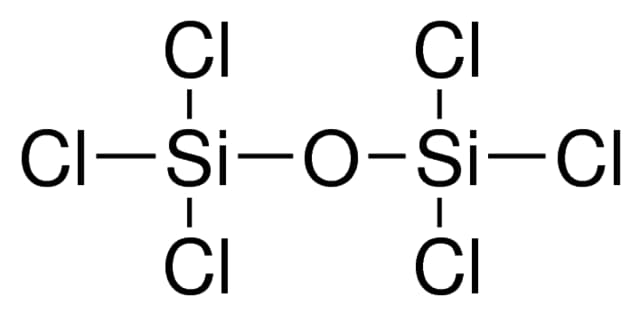
Hexachlorodisiloxane, also known as HCDSO or Hexachloropropanedisiloxane, is an organosilicon compound with the molecular formula Cl₆OSi₂ and a molecular weight of 284.89 g/mol. Identified as a colorless liquid, it has a purity level exceeding 98%, verified through elemental analysis. Due to its moisture sensitivity, it requires storage in tightly sealed containers, ideally under an inert atmosphere, to prevent decomposition.
Structural and Chemical Characteristics
The molecular structure of hexachlorodisiloxane features two silicon atoms linked by an oxygen bridge, with each silicon further bonded to three chlorine atoms. This configuration provides the compound with a high degree of reactivity, particularly in reactions involving hydrolysis or chlorination. Hexachlorodisiloxane’s boiling point is recorded at 137°C, which is significant for handling and storage at controlled temperatures, ensuring stability during use.
Applications in Synthesis and Industry
Hexachlorodisiloxane is commonly utilized as a precursor in organosilicon chemistry and as a reagent in the synthesis of chlorosilane-based compounds. Its structure makes it especially valuable in producing various silicon-based intermediates, which are integral to the development of silicone materials, resins, and coatings. Additionally, hexachlorodisiloxane is frequently used in high-purity applications that demand stringent control over impurities and reactivity.
Safety and Handling Precautions
Classified with hazard statements H225, H314, H318, and H335, hexachlorodisiloxane is both flammable and corrosive, warranting caution during handling. Adequate ventilation, along with protective gloves and eyewear, is recommended to prevent contact with skin or eyes. Its flammability and boiling point necessitate storage away from ignition sources and under conditions that minimize exposure to atmospheric moisture, thereby ensuring compound integrity.
Relevance in Industrial and Laboratory Settings
In both industrial and research environments, hexachlorodisiloxane is essential for processes that require reactive chlorosilane intermediates. Its role as a precursor supports the synthesis of advanced materials with high-performance characteristics, particularly in applications requiring precise chemical control and stability.
Hexachlorodisiloxane, reacting with primary and secondary amines leads, dependent on stoichiometry, to numerous partially and totally organylamino substituted disiloxanes. Partially amino-substituted chlorodisiloxanes are very sensitive to moisture and can be converted into disiloxanes with different organylamino groups. Exhaustive alkanolysis substitutes amino as well as chloro groups giving hexaalkoxydisiloxanes, but partial alkanolysis may substitute amino in preference to chloro-groups. See links below
Ereztech manufactures and sells this product in small and bulk volumes. Glass ampules, bottles or metal ampules or bubblers are available for packaging. For additional analytical information or details about purchasing SI6211 contact us at sales@ereztech.com
Safety information
| UN | 2985 |
| Hazardous class | 3(8) |
| Packing group | II |
| Pictograms |   |
| Signal word | DANGER |
| Hazard statements | H225-H314-H318 |
| Precautionary statements | P210-P233-P240-P241-P242-P243-P260-P264-P271-P280-P301 + P330 + P331-P303 + P361 + P353-P304 + P340-P305 + P351 + P338-P310-P363-P403 + P233-P405-P501 |
| Transport description | Chlorosilanes, corrosive, n.o.s. (Hexachlorodisiloxane) |
| In TSCA registry | Yes |
First Aid Measures
| General advice | Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of the dangerous area. |
| Eye contact | Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. |
| Skin contact | Wash off with soap and plenty of water. Consult a physician. |
| Inhalation | If breathed in, move person into the fresh air. If not breathing, give artificial respiration. Consult a physician. |
| If swallowed | Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. |
Certificates of Analysis (CoA)
If you don’t see the needed lot of Hexachlorodisiloxane below please contact customer support at sales@ereztech.com
Lot# 2P21020 Lot# 2R05720 Lot# 12R4.10220 Lot# 12R50220
External identifiers for HCDSO
| Pubchem CID | 84745 |
| IUPAC Name | trichloro(trichlorosilyloxy)silane |
| SMILES | O([Si](Cl)(Cl)Cl)[Si](Cl)(Cl)Cl |
| InchI Identifier | InChI=1S/Cl6OSi2/c1-8(2,3)7-9(4,5)6 |
| InchI Key | QHAHOIWVGZZELU-UHFFFAOYSA-N |
Known applications and external links
- Ulrich Wannagat, Gabriele Bogedain, Hamid Hajibegli, Hans-Heinrich Moretto Beiträge zur Chemie der Silicium-Stickstoff-Verbindungen, 166. Mitt.: Organylaminosubstitutionen an Hexachlordisiloxan
- I. S. Ignat’ev, A. N. Lazarev Normal vibrations and force field of hexachlorodisiloxane Cl3SiOSiCl3
With Silicon tetraiodide oxide other customers often ask:
Ereztech synthesizes and sells additional SI-compounds.
To purchase SI6211 contact us at sales@ereztech.com



