Silicon(IV) bromide
Synonym: Tetrabromosilane, Silicon tetrabromide, Silicon bromide, SiBr4
CAS Number 7789-66-4 | MDL Number MFCD00049530 | EC Number 232-182-4
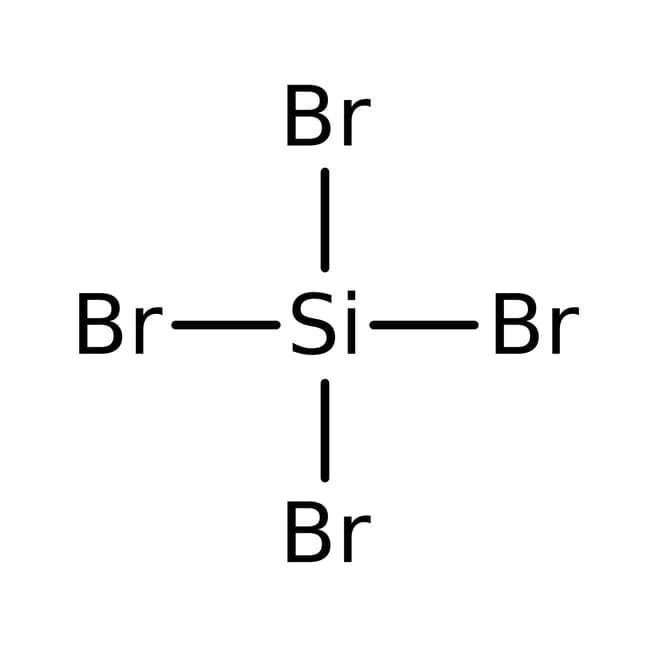
Silicon(IV) bromide, also known as tetrabromosilane or silicon tetrabromide, is a colorless liquid with the molecular formula SiBr₄ and a molecular weight of 347.70 g/mol. This compound consists of one silicon atom covalently bonded to four bromine atoms, forming a tetrahedral structure. It is a highly reactive inorganic compound that is widely used in various chemical processes. Silicon(IV) bromide has a melting point of approximately -15 °C and a boiling point of around 153 °C, making it a liquid at room temperature.
The appearance of silicon bromide is described as a colorless liquid, and it is sensitive to moisture, meaning it should be handled and stored in dry conditions to prevent decomposition. Silicon(IV) bromide is known for reacting readily with water, producing silicon dioxide and hydrobromic acid in a highly exothermic reaction. Therefore, proper storage conditions, typically in a tightly sealed container and away from water sources, are essential for maintaining the stability of the compound.
This compound is primarily used in the chemical vapor deposition (CVD) process, where it serves as a precursor for producing high-purity silicon-based thin films. These films are critical components in the semiconductor industry, particularly in the production of microchips and other electronic devices. Additionally, silicon bromide is used in organic synthesis and as a reagent in laboratory settings, making it valuable for research and development purposes.
In terms of health and safety, silicon tetrabromide can be hazardous when in contact with skin, eyes, or when inhaled. It can cause severe irritation and burns due to its reactive nature, especially in the presence of moisture. Thus, handling this compound requires protective equipment such as gloves, goggles, and respirators to avoid direct exposure. Furthermore, proper ventilation is necessary in environments where silicon bromide is used, to prevent inhalation of fumes.
Silicon(IV) bromide is a highly reactive, moisture-sensitive compound with critical applications in chemical processes like CVD and organic synthesis. Its role as a precursor in the semiconductor industry underlines its importance in the production of high-tech devices. Proper handling, storage, and safety precautions are crucial when working with this substance to avoid hazardous reactions and health risks.
Silicon(IV) bromide due to it’s close similarity to Silicon Tetrachloride has several unique features, applicable in the production of ultrapure silicon for the manufacture of semiconductor devices. This product has an advantage over SiCl4 in the speed of silicon deposition. Pyrolysis of Silicon(IV) bromide followed by treatment with ammonia yields Silicon Nitride (Si3N4) coatings. This hard compound is used for ceramics, sealants, and the production of many cutting tools. It is also used in water treatment, chemical analysis and in ultra high purity for certain crystal growth applications. Synthesis of this product has been reported (see the links below).
Ereztech manufactures and sells of Silicon(IV) bromide in small and bulk volumes. Glass ampules and bottles or metal ampules and bubblers are available for packaging. For additional information or details about purchasing Silicon(IV) bromide contact us at sales@ereztech.com
Safety information
| UN | 3264 |
| Hazardous class | 8 |
| Packing group | II |
| Pictograms |  |
| Signal word | DANGER |
| Hazard statements | H314-H318 |
| Precautionary statements |
P260-P264-P280-P301 + P330 + P331-P301 + P330 + P331-P303 + P361 + P353-P304 + P340-P305 + P351 + P338- P310-P403 + P233 + P235-P405-P501 |
| Transport description | CORROSIVE LIQUID, ACIDIC, INORGANIC, N.O.S. (Silicon(IV) bromide) |
| In TSCA registry | Yes |
First Aid Measures
| General advice | Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of dangerous area. |
| Eye contact | Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. |
| Skin contact | Wash off with soap and plenty of water. Consult a physician. |
| Inhalation | If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. |
| If swallowed | Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. |
Certificates of analysis (CoA)
If you don’t see the needed lot of Silicon(IV) bromide below please contact customer support at sales@ereztech.com
Lot# 005/613 Lot# 234RP2Si56419
External identifiers for Tetrabromosilane
| Pubchem CID | 82247 |
| SMILES | Br[Si](Br)(Br)Br |
| IUPAC Name | Silicon tetrabromide |
| InchI Identifier | InChI=1S/Br4Si/c1-5(2,3)4 |
| InchI Key | AIFMYMZGQVTROK-UHFFFAOYSA-N |
Known applications and external links
- Schumb, W. B. Silicobromoform” Inorganic Syntheses 1939, volume 1, pp 38-42
- Silicon Compounds, Inorganic. Simmler W.; Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH, 2002
With Silicon(IV) bromide other customers often ask:
Ereztech synthesizes and sells additional Si-compounds.
To purchase Silicon(IV) bromide contact us at sales@ereztech.com



